Electrochemical Characterization of Amorphous and Crystalline Ni62Nb38 and Ni59.24Nb37.76B3.00 Alloys
DOI:
https://doi.org/10.14295/vetor.v34i1.17711Keywords:
Amorphous Ni-Nb-based Alloys, Electrochemical characterization, Corrosion resistanceAbstract
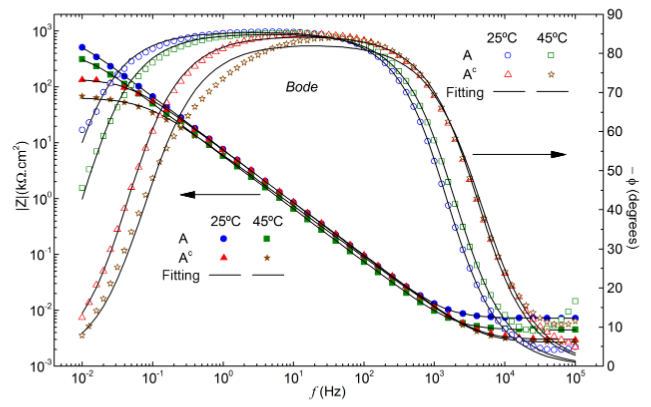
Nickel-based alloys are widely used in industry due to their remarkable corrosion resistance. Currently, most of these alloys are processed with crystalline structure. However, amorphous metal alloys commonly demonstrate greater corrosion resistance compared to their crystalline counterparts. In this study, the Ni62Nb38, Ni59.24Nb37.76B3.00, and Ni58.1Nb38.9B3.0 (atom percent) alloys with crystalline and amorphous structure were investigated. Traditional X-ray diffraction (XRD) and differential scanning calorimetry (DSC) techniques were used to characterize the alloys. Electrochemical tests were conducted to evaluate the corrosion resistance at different temperatures. Data obtained by electrochemical impedance spectroscopy and polarization curves revealed the superiority of amorphous alloys in relation to crystalline ones, for the same chemical composition. The polarization resistance of the amorphous alloys was up to 20 times greater than that of its crystalline counterparts. Both structures showed a reduction in corrosion resistance with increasing temperature. In amorphous alloys, the presence of boron made the samples more resistant to corrosion at both temperatures. Furthermore, a higher percentage of niobium among the ternary compositions also improved the corrosion properties. In crystalline alloys, the presence of boron resulted in samples that were less resistant to corrosion at a temperature of 25 °C. However, this element provided greater resistance to ternary alloys at a temperature of 45 °C. Using electrochemical techniques, it was possible to demonstrate the superior corrosion resistance of amorphous alloys compared to its crystalline counterparts.
Downloads
References
J. S. Andrade, I. N. Bastos, and L. C. R. Aliaga, “Determinação das características estruturais e mecânicas da liga de alta entropia Hf-Nb-Ta-Zr,” Vetor, vol. 30, no. 2, pp. 22–32, 2021. Available at: https://doi.org/10.14295/vetor.v30i2.13090
K. Zheng, Z. He, L. Che, H. Cheng, M. Ge, T. Si, and X. Xu “Deep alloys: Metal materials empowered by deep learning,” Materials Science in Semiconductor Processing, vol. 179, p. 108514, 2024. Available at: https://doi.org/10.1016/j.mssp.2024.108514
S. Xie, J. Zhao, S. Li, and J. Su, “Enhanced mechanical properties of Zr-Cu-Al-Ni bulk amorphous alloys by Ag and O doping,” Journal of Alloys and Compounds, vol. 957, p. 170186, 2023. Available at: https://doi.org/10.1016/j.jallcom.2023.170186.
H. Sonomura, K. Katagiri, T. Ozaki, Y. Hasegawa, T. Tanaka, and A. Kakitsuji, “In-situ preparation of Zr-Al-Ni-Cu amorphous alloy by friction stirring using a tool consisting of multiple metal foils,” Materials Letters, vol. 353, p. 135264, 2023. Available at: https://doi.org/10.1016/j.matlet.2023.135264
S. F. Guo, H. J. Zhang, Z. Liu, W. Chen, and S. F. Xie, “Corrosion resistances of amorphous and crystalline Zr-based alloys in simulated seawater,” Electrochemistry Communications, vol. 24, pp. 39–42, 2012. Available at: https://doi.org/10.1016/j.elecom.2012.08.006
H. Zhang, C. Zhang, B. Han, J. Qiu, H. Li, S. Qin, J. Liu, Y. Wang, P. Zhang, Y. Pan, and H. Zhou “Evolution of grain boundary character distributions in a cold-deformed Nickel-based superalloy during electropulsing treatment,” Journal of Materials Research and Technology, vol. 9, no. 3, pp. 5723–5734, 2020. Available at: https://doi.org/10.1016/j.jmrt.2020.03.097
C.-Y. Lee, T.-J. Lin, H.-H. Sheu, and H.-B. Lee, “A study on corrosion and corrosion-wear behavior of Fe-based amorphous alloy coating prepared by high velocity oxygen fuel method,” Journal of Materials Research and Technology, vol. 15, pp. 4880–4895, 2021. Available at: https://doi.org/10.1016/j.jmrt.2021.10.103
H. Alves and U. Heubner, “Aqueous Corrosion of Nickel and its Alloys,” in Shreir's Corrosion, vol. 3, 2010, pp. 1879–1915. Elsevier eBooks. Available at: https://doi.org/10.1016/b978-044452787-5.00092-5
C. Suryanarayana and A. Inoue, Bulk Metallic Glasses, 2nd edition. 2017.
L. Xia, W. H. Li, S. S. Fang, B. C. Wei, and Y. D. Dong, “Binary Ni–Nb bulk metallic glasses,” Journal of Applied Physics, vol. 99, no. 2, 2006. Available at: https://doi.org/10.1063/1.2158130
W. J. Botta, J. E. Berger, C. S. Kiminami, V. Roche, R. P. Nogueira, and C. Bolfarini, “Corrosion resistance of Fe-based amorphous alloys,” Journal of Alloys and Compounds, vol. 586, pp. S105–S110, 2014. Available at: https://doi.org/10.1016/j.jallcom.2012.12.130
G.Y. Koga, R.P. Nogueira, V. Roche, A.R. Yavari, A.K. Melle, J. Gallego, C. Bolfarini, C.S. Kiminami, W.J. Botta, “Corrosion properties of Fe–Cr–Nb–B amorphous alloys and coatings,” Surface & Coatings Technology, vol. 254, pp. 238–243, 2014. Available at: https://doi.org/10.1016/j.surfcoat.2014.06.022
A. Fattah-Alhosseini, F. Soltani, F. Shirsalimi, B. Ezadi, and N. Attarzadeh, “The semiconducting properties of passive films formed on AISI 316 L and AISI 321 stainless steels: A test of the point defect model (PDM),” Corrosion Science, vol. 53, no. 10, pp. 3186–3192, 2011. Available at: https://doi.org/10.1016/j.corsci.2011.05.063
K. Park, S. Ahn, and H. Kwon, “Effects of solution temperature on the kinetic nature of passive film on Ni,” Electrochimica Acta, vol. 56, no. 3, pp. 1662-1669, 2011. Available at: https://doi.org/10.1149/ma2012-02/22/2179
C. Qin, W. Zhang, H. Kimura, K. Asami, and A. Inoue, “New Cu-Zr-Al-Nb Bulk Glassy Alloys with High Corrosion Resistance,” Materials Transactions, vol. 45, no. 6, pp. 1958–1961, 2004. Available at: https://doi.org/10.2320/matertrans.45.1958
S.-J. Pang, C.-H. Shek, T. Zhang, K. Asami, and A. Inoue, “Corrosion behavior of glassy Ni55Co5Nb20Ti10Zr10 alloy in 1N HCl solution studied by potentiostatic polarization and XPS,” Corrosion Science, vol. 48, no. 3, pp. 625–633, 2006. Available at: https://doi.org/10.1016/j.corsci.2005.02.013